St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Aditi Bagchi, MD, PhD
Using genetic and molecular features of infantile brain tumors to inform clinical risk stratification and targeted therapies
Overview
As our understanding of brain tumor biology improves, so does our ability to treat them. My clinical research focuses on using the molecular profiles of pediatric brain tumors to categorize them into risk groups and develop targeted. My approach aims not only to tailor brain tumors to improve survival outcomes but to also reduce the long-term effects associated with cancer therapy, leading to improved quality of life in survivors.

Bagchi Research Summary
Infants with brain tumors (age 0-3) are some of the most vulnerable patients we treat. Because of the early stages of central nervous system development, infants are often prone to significant cognitive impairment, developmental delays, endocrine deficiencies and growth failure as a consequence of cancer-directed therapies like radiation and chemotherapy. Therefore, it is imperative to choose effective, targeted treatments to mitigate morbidity associated with treatment while improving survival rates.
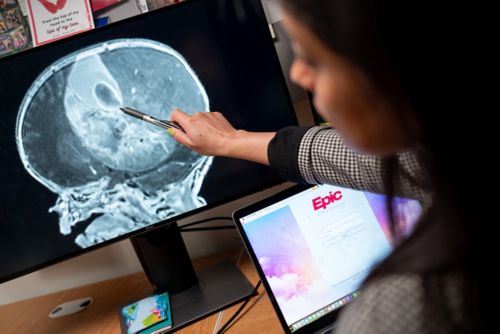
Medulloblastoma in infants
Utilizing this molecularly informed treatment strategy guides my approach as co-principal investigator of SJiMB21. SJiMB21 is a phase 2 clinical trial that uses molecular and clinical risk-based therapeutic approaches for newly diagnosed medulloblastoma in infants (0-3 years) and young children (3-5 years). This is the first clinical protocol to use DNA methylation-based classification to guide treatment for pediatric brain tumors.
Medulloblastoma can be classified into four molecular groups (WNT, SHH, G3 and G4) and 13 molecular subgroups (WNT, SHH-1, SHH-2, SHH-3, SHH4, G3/G4-1, G3/G4-2, G3/G4-3, G3/G4-4, G3/G4-5, G3/G4-6, G3/G4-7 and G3/G4-8) based on methylation profiles. Retrospective research has shown that the most common group and subgroup of medulloblastoma in infants are SHH-1 and SHH-2, which can be effectively treated without radiation. Meanwhile, the molecular group G3/G4 is much rarer and cannot be cured without radiation.
SJiMB21 uses methylation-based classification to profile medulloblastoma risk and stratify and assign treatment based on molecular group/subgroup. Using this approach, we design targeted treatment approaches that spare SHH patients of radiation. We treat SHH-2 patients with lower intensity systemic chemotherapy while SHH-1 patients receive a more intensified chemotherapy and G3/G4 patients are given delayed, risk-adapted radiation.
High-Grade Gliomas in infancy
Among the brain tumors diagnosed during infancy, gliomas are the most common. Pediatric high-grade gliomas (pHGG) are diagnostically and clinically challenging during infancy. pHGGs have a better prognosis in infants, but predicting individual outcomes is elusive.
In a study comprising 56 infants with pHGG, it was identified that infant type hemispheric glioma (IHG) is a type of pHGG that is volumetrically large and hemorrhagic but primarily drives the better prognosis in this age group. Molecular studies of IHG have shown distinct methylation profiles and genetic drivers that consist of receptor tyrosine kinase (RTK) fusion (ALK, ROS1, NTRK1/2/3, and MET). The RTK fusions can be targeted by unique tyrosine kinase inhibitors that cause rapid and sustained shrinkage of these tumors, making them safe for surgical resection.
As a next step in this work, I am developing a frontline clinical trial that will test the use of the tyrosine kinase inhibitor as primary therapy. The objective of this trial is to improve the survival of patients but also mitigate the toxicities of chemotherapy and aggressive surgeries that are currently being used to treat these tumors.
A large part of my clinical research involves seeing patients in the St. Jude Brain Tumor Clinic. I see about 25 new patients per year and serve as a consultant across the U.S. for patients with rare infantile brain tumors. Each opportunity to care for a patient informs the next step in clinical research as we seek to improve survival rates and quality-of-life measures for patients with infantile brain tumors.
Selected Publications
About Aditi Bagchi
Dr. Aditi Bagchi is an Associate Member in the Department of Oncology, Division of Neuro-Oncology. As a pediatric neuro-oncologist, Dr. Bagchi uses a translational approach, bringing novel insights from the laboratory and clinical research studies into the clinic. As a physician with a clinical and research background — she received her PhD in Cell and Molecular Biology and completed a residency and fellowships in Pediatrics, Pediatric Hematology/Oncology and Pediatric Neuro-Oncology — Dr. Bagchi possesses foundational expertise that allows her to evaluate the biological and molecular differences in brain tumors to develop a risk-stratified treatment approach. Much of Dr. Bagchi’s work focuses on assessing therapeutic approaches for infants with high-grade glioma in the hope of improving outcomes and reducing long-term morbidity.

Contact us
Aditi Bagchi, MD, PhD
Associate Member, St. Jude Faculty
Department of Oncology
Division of Neuro-Oncology
MS260, Room C6057
St. Jude Children's Research Hospital

Memphis, TN, 38105-3678 USA GET DIRECTIONS