St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Zebrafish teach us how to make blood stem cells

Wilson Clements, PhD, and Erich Damm, PhD, used time-lapse videos to find cells that signal future blood stem cells to become stem cells.
How did a pet store fish—the zebrafish—help bring us a giant step closer to understanding how to make perfect blood stem cells? Here’s how.
What are blood stem cells?
My group and dozens of other groups around the world have been trying to learn how to make perfect blood stem cells for many years. Blood stem cells are used to treat patients who have blood diseases, such as leukemia, sickle cell disease, congenital neutropenias and thalassemias. For those patients, the idea is to get rid of the “bad” blood and replace it with new, “good” blood.
Rare blood stem cells live in the bone marrow, where they occasionally divide. Sometimes they make a new blood stem cell, and sometimes they make a daughter that can proliferate the huge number of times needed to replace 230 billion blood cells every day. Because blood stem cells self-renew, they can fully restore all of the red (oxygen-carrying) and white (immune) blood cells needed by a patient. Forever.
But there’s a catch. White blood cells attack anything foreign—if you transplant stem cells that are not almost exactly identical to the patient who gets them, they will produce white blood cells that attack the recipient. Instead of the patient rejecting the transplant, the transplant rejects the patient, resulting in graft versus host disease.
Our solution? Make perfect blood stem cells in a petri dish.
Making Perfect Blood Stem Cells
We want to learn how to make perfect stem cells. And we learn from the best teacher available, the embryo. When embryos develop, they make every kind of cell a person could need: eye cells, skin cells, liver cells, kidney cells, everything—including blood stem cells.
The very first fertilized cell divides again and again. The new cells specialize to eventually make the roughly 37 trillion cells found in an adult human. Some cells contribute to the brain, some to the stomach and some to blood. As the embryo develops, these cells “talk” to each other with protein signals and guide one another in eventually realizing their adult fates.
The key to replicating the process of blood stem differentiation in a petri dish is to be able to create a single cell with the potential to become any kind of adult cell, then treat it as it divides with the right sequence of signals to guide it and its daughter cells to becoming a blood stem cell. For this, we turn to the zebrafish.
Learning from the Zebrafish
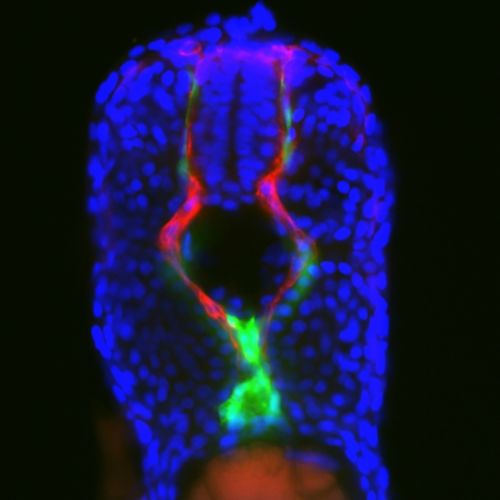
Researchers used strategies like time-lapse video to identify cells that communicate with the future blood stem cells and direct them to become blood cells. The cells involved migrate during development. These results will likely fuel new types of research in stem cell biology and blood development.
The zebrafish is a small pet store fish that originally came from the Ganges and other Himalayan river drainages in India. It has some special qualities that make it ideal for learning about embryonic development.
For one thing, it doesn’t develop inside a uterus. It’s transparent during the times when it forms organs, brain and blood. To see these tissues get made, just look under a microscope.
Also, the zebrafish blood system and the way it gets made are incredibly similar to humans, so we have been able to learn many things that can be directly applied to human health from this teacher. Zebrafish have all eight kinds of adult red and white blood cell types humans have, and the processes that make those first blood stem cells in the embryo seem to be almost entirely identical. Nearly every time researchers have found a protein signal important in zebrafish blood stem cell differentiation, that same signal has turned out to be important in humans.
Researchers now have many special varieties of zebrafish where particular types of cells are fluorescent. Nerve cells can be seen through a microscope, as they glow red or green. Fish with fluorescent blood and blood vessels can also be studied.
Brain Talks to Blood
Blood stem cells come from special cells in the developing blood vessels. Until now, no one has known the origin of neighboring cells that probably provide the necessary signals to tell them to become blood stem cells.
Using the zebrafish, we made a remarkable discovery: some of the neighbor cells come from far away in the developing spinal cord. These future nerve cells crawled out and traveled down to the blood vessel, ending up right next to the future blood stem cells. Using the power of fluorescent zebrafish, we could directly see these cells make their journey and very first contact. And when we prevented those nerves from reaching their target? No blood stem cells.
We don’t know what special signal or signals these nerves contain, but now we know where it lives, and that brings us one big step closer.
You can read about these results in our latest paper in Nature Cell Biology.






