St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
About the Feng Lab
Immune tolerance is a finely tuned state of immune unresponsiveness that prevents the immune system from mistakenly attacking the body’s own tissues, food and commensal microbiome. This process is essential for preventing autoimmune diseases and allergies as well as maintaining immune balance. Regulatory T (Treg) cells are long-lived suppressor T cells that play a crucial role in immune tolerance and homeostasis throughout life. Our research bridges basic immunology and preclinical models to uncover how immune suppressive Treg cells interact with inflammatory T cells and tissue cells. We also explore how Treg cells can be harnessed or reprogrammed to treat autoimmune diseases, cancer, and chronic inflammation.
Our research summary
We employ cutting-edge technologies, such as novel preclinical models, genome editing, structure-guided protein design, single-cell multiomics and computational analysis to investigate how Treg cells are programmed by environmental and genetic factors through signaling pathways, epigenetic modifications and transcriptional regulation. These processes establish and maintain immunosuppressive memory toward self-antigens, foreign antigens and neoantigens. Conversely, we study how disruptions in these processes lead to immune-related diseases or how tumors exploit them to evade immune attack. We also leverage this knowledge to explore the therapeutic potential of Treg cells through synthetic biology approaches.
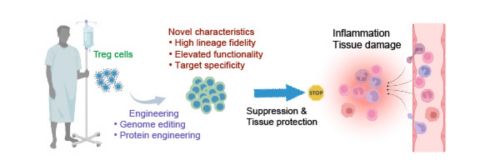
Genome editing and protein engineering are employed to modify Treg cells to achieve high lineage fidelity, elevated functionality, or selected target specificity for basic and translational research. These Treg cells can be used to suppress autoimmune inflammation, tissue damage, or degeneration.
Treg lineage stability and long-term tolerance
Treg cells are long-lived, but how their lineage stability is maintained remains a key research question. Investigating regulation of the Treg master regulator, Foxp3, provides a simple yet powerful approach to address this question. Specific DNA regions act like switches to keep Foxp3 active. If these switches fail, Treg cells lose their identity and stability, leading to widespread inflammation. Our lab is exploring how these mechanisms operate in health and disease, and how we might modulate them to enhance or reduce Treg stability for therapeutic benefit.
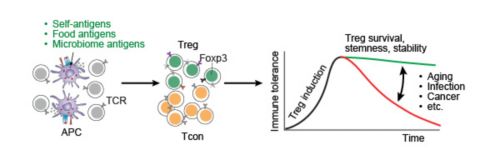
The induction of regulatory T (Treg) cells contributes to the non-responsiveness (immune tolerance) of conventional T (Tcon) cells. The maintenance of immune tolerance depends on the survival, stemness, and lineage stability of Treg cells. They may be affected by various factors, such as aging, infection, and cancer, which is currently being extensively investigated.
Treg diversity and suppression strategies
Treg cells are induced in the thymus or peripheral tissues in response to self-antigens or foreign antigens presented by specialized antigen-presenting cells (APCs), such as medullary thymic epithelial cells (mTECs). This tolerance memory is established during the initial encounter with these antigens and is crucial for selectively suppressing T-cell reactivity towards specific targets, including self-organs, food antigens and the commensal microbiome.
Treg induction involves a complex interplay between specialized APCs and Treg precursors through both extrinsic and intrinsic mechanisms. Our studies show that Treg development is guided by key genetic programs that shape their specificity and function. Disruption of these programs can lead to outcomes ranging from mild immune dysregulation to fatal systemic autoimmunity. Our work provides insights into how Treg cells adapt to different targets and how their suppression strategies shift.

Gene regulation in T-cell tolerance
We are also exploring how immune tolerance is established through gene regulation in both Treg cells and conventional T cells. Using cutting-edge epigenetic and proteomics tools, we discovered that Foxp3 does not function as a typical transcription factor but rather as a transcriptional cofactor. This seemingly minor reclassification of Foxp3 leads to a profoundly different comprehension of how it regulates gene expression to determine the fate and function of Treg cells.
We are investigating the association of Foxp3 with other DNA-binding proteins, such as NFAT, to address the nature of Foxp3-DNA and Foxp3-chromatin interactions. We are also elucidating the key determinants of dynamic Foxp3-dependent gene regulation and the immunological contexts that rely on these mechanisms
Contact us
Yongqiang Feng, PhD
Associate Member, St. Jude Faculty
Department of Immunology
MS351, Room M5422
St. Jude Children’s Research Hospital
Join us
We welcome motivated scientists to join our team! Please visit the St. Jude Careers page to explore current opportunities or contact Dr. Feng directly.

Memphis, TN, 38105-3678 USA GET DIRECTIONS