St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Thirumala-Devi Kanneganti Lab
Providing fundamental insights into innate immunity, including inflammasomes and inflammatory cell death, PANoptosis, to discover therapeutic targets in infectious and inflammatory diseases and cancer.
About the Kanneganti Lab
Innate immunity is the first line of defense against disease. Sensing pathogens and homeostatic disruptions leads to signaling in innate immune and barrier cells that drives inflammation and cell death to combat infection or tumorigenesis. However, aberrant, excessive or chronic inflammation and cell death can contribute to the development of many diseases including autoinflammatory and autoimmune disorders and cancer. Our laboratory seeks to gain fundamental insights into innate immune mechanisms to identify new processes, sensors and signaling molecules. This will inform the development of novel therapeutic strategies.

Our research summary
Our laboratory is known for fundamental discoveries elucidating functions of innate immune sensors, inflammasomes, and inflammatory cell death. Inflammation and cell death are key processes in host defense and the elimination of aberrant cells. Cell death pathways have historically been investigated using individual stimuli. However, this does not reflect the complexity in the body, where cells are constantly exposed to multiple stimuli that can be detected by innate immune sensors to drive inflammatory cell death. Using multidisciplinary approaches, our lab works to understand this complexity and identify the molecular mechanisms of innate immune sensing in cell death and disease to open new frontiers in research. Our work has identified extensive crosstalk among cell death molecules. Building on our findings, we pioneered the concept of PANoptosis, an innate immune, inflammatory, and lytic cell death pathway. Our lab continues to pursue studies that define the role of innate immune sensors and cell death molecules in health and disease.

Inflammasomes
Our lab has contributed to both the inception and maturation of the inflammasome field as a major research area in immunology and inflammation research. An inflammasome is a multimeric protein complex that is a critical component of the innate immune response. Inflammasomes form when certain innate immune sensors detect pathogen- or damage-associated molecular patterns. This complex formation drives the maturation and release of inflammatory IL-1 family cytokines, IL-1β and IL-18. These cytokines can be beneficial in host defense but can also lead to aberrant inflammation.
As a founding member of the inflammasome field, we provided the first genetic evidence for the role of the innate immune sensor, NLRP3, in microbial-mediated inflammasome activation and established the importance of the NLRP3 inflammasome in intestinal inflammation, neuroinflammation, cancer, and metabolic diseases. In parallel, we have identified the activation mechanisms of other inflammasomes including NLRC4, NLRP1, PYRIN, and AIM2 in infection, inflammatory disease, and cancer. Additionally, we have characterized distinct, novel roles for IL-1 family cytokines (IL-1α, IL-1β, and IL-33) in disease. We continue to investigate these molecules and their roles in health and disease.

PANoptosis
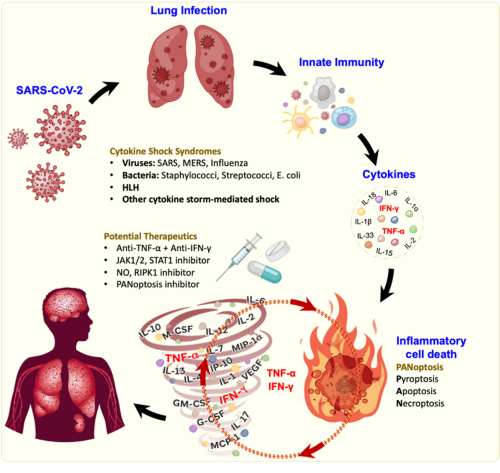
Our studies on inflammasomes led us, and others, to perform genetic, molecular and biochemical studies that identified extensive crosstalk among cell death molecules. Our work on these molecules established new paradigms connecting innate immune sensing to cell death and disease, advancing our understanding of pathway regulation and coordination during infection and inflammation. These findings led our lab to pioneer the paradigm-shifting concept of PANoptosis – a prominent innate immune, inflammatory cell death pathway initiated by innate immune sensors and driven by caspases and RIPKs through multi-protein PANoptosome complexes. Inflammasomes can also be integral components of these complexes.
We identified ZBP1 as the first innate immune sensor to form a PANoptosome and induce PANoptosis. Building on this initial discovery, our work continues to elucidate pathogens, cytokine signaling pathways, and inflammatory syndromes that activate PANoptosis, implicating this process in infectious and autoinflammatory diseases, cancer, and beyond. To date, we have identified multiple PANoptosome complexes, including the ZBP1-, NLRP3-, NLRC5- and NLRP12-, RIPK1-, and AIM2-PANoptosomes, and described their roles in disease. Furthermore, we made transformative contributions to the cytokine field, defining the mechanistic basis for cytokine storm. Overall, PANoptosis is now implicated in driving innate immune responses and inflammation across the disease spectrum, and our lab is continuing to investigate the physiological roles of this process.
Ongoing research in our lab continues to focus on innate immunity, inflammasomes, and cell death in physiologically relevant conditions, with the goal of translating mechanistic insights into therapeutic opportunities. With more than 370 manuscripts, our fundamental work continues to reshape the way we understand innate immune cell death pathways, inform the development of therapeutics, and advance our understanding of innate immunity and inflammation.
Selected Publications
Contact us
Thirumala-Devi Kanneganti, PhD
Department of Immunology
MS351
St. Jude Children's Research Hospital
Memphis, TN, 38105-3678 USA 901-595-3634 Thirumala-Devi.Kanneganti@stjude.org

Memphis, TN, 38105-3678 USA GET DIRECTIONS