St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Missed connection no more: Science and serendipity solve a microRNA mystery
Those with beta-thalassemia have less blood cells, caused by the toxic buildup the alpha-globin protein.
The scientist Louis Pasteur said that “chance favors the prepared mind.” That’s precisely what occurred when two distinct research paths by different scientists in the same St. Jude laboratory intertwined unexpectedly, making pieces of a long-standing puzzle fall into place. What they uncovered was an unexpected connection between a highly expressed red blood cell microRNA gene and potential new treatments for beta-thalassemia, a common genetic anemia. MicroRNAs are a mechanism to fine-tune protein expression according to physiological stresses, making them a compelling place to look for therapeutic opportunities.
St. Jude researcher Mitchell J. Weiss, MD, PhD, St. Jude Department of Hematology chair and Christophe Lechauve, PhD, formerly a postdoctoral fellow and Research Associate in Dr. Weiss’ lab and now a Principal Scientist at Sanofi, were trying to understand how cellular protein quality control systems can lessen the severity of beta-thalassemia. They recognized a potential connection with a different project focused on understanding the functions of miR-144/451, a microRNA gene that is abundantly expressed during red blood cell development. As the two projects proceeded, they intertwined to reveal a previously underappreciated connection between miR-144/451, metabolic regulations and beta-thalassemia, according to a recently published story in Blood.
“After studying beta-thalassemia and miR/144/451 separately in my laboratory for more than 15 years, we found this unexpected connection,” said Weiss, a co-corresponding author on the paper.
Finding a connection between beta-thalassemia, microRNAs and metabolism
Beta-thalassemia results from mutations in the beta-globin gene that reduce or eliminate red blood cell’s ability to produce the beta subunit of hemoglobin, a critical molecule that transports oxygen from the lungs to all tissues. Hemoglobin comprises alpha and beta globin protein subunits. In patients with untreated beta-thalassemia, surplus alpha-globin protein accumulates within red blood cells and their precursors, leading to complications such as severe anemia, stunted growth, fatigue and other conditions related to an accumulation of a toxic protein and a lack of oxygen transport to tissues.
Weiss, Lechauve and their colleagues discovered that a single gene expressing two different microRNAs (miR-144 and miR-451) regulates two distinct metabolic pathways involved in clearing the buildup of harmful alpha-globin; this finding may aid the development of future treatments for beta-thalassemia. Understanding how this microRNA interacts with beta-thalassemia is the serendipitous product of 15 years of research.
“MiR-144/451 is distinctive because it is by far the most highly expressed microRNA gene in red blood cells,” said Lechauve, the lead co-corresponding author, who led many of the experiments in the study. “Disruption of the miR-144/451 gene in otherwise healthy mice causes mild impairments in the production of red blood cells and their lifespan in the circulation. However, we were surprised to find that loss of miR-144/451 has the opposite effects in beta-thalassemic mice, where it improved red blood cell production and lifespan. By studying this novel genetic interaction, we learned new things about the function of miR-144/451 and the metabolic pathways that regulate protein quality control in beta-thalassemia.”
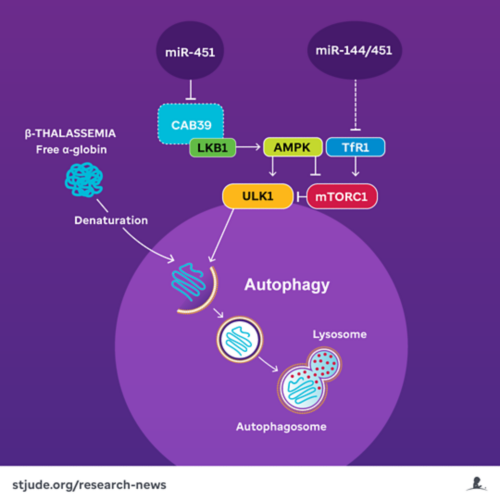
MicroRNAs 144/451 block autophagy of alpha-globin through multiple metabolic pathways involving mTORC1.
The team investigated a metabolic pathway associated with alpha-globin degradation in a beta-thalassemia mouse model. They found that two major metabolic proteins, mTORC1 and AMPK, are responsible for determining if a third protein, ULK1, could initiate the autophagy of free alpha-globin. Autophagy is a process that degrades and recycles unnecessary or potentially toxic cellular proteins, including surplus alpha-globin. The scientists’ previous research had hinted at the potential for inhibiting mTORC1, which typically prevents autophagy, as a treatment for beta-thalassemia in mice, as it would lead to greater alpha-globin recycling.
In a different project, investigators in Weiss’ lab and Duonan Yu, a former postdoctoral fellow who is now a Professor at Yangzhou University, showed that miR-451 inhibits AMPK, a negative regulator of mTORC1.
“We reasoned that loss of miR144-451 might therefore activate mTORC1 by stimulating AMPK, causing enhanced autophagy of free alpha-globin,” said Lechauve. With this in mind, the researchers disrupted the miR-144/451 gene in beta-thalassemic mice. When they knocked out the microRNA, they could hardly believe its impact.
“The absence of this microRNA gene in beta-thalassemic mice caused an 80-90% reduction in the accumulation of toxic free alpha-globin,” Lechauve said. “The effect was major. Our previous attempts at stimulating the autophagy of free alpha-globin resulted in only 30-40% reduction. In addition to the large decrease in toxic protein buildup, we saw that the production of red blood cells and their circulating lifespan improved in our beta-thalassemia model.”
A serendipitous find: Second mechanism of microRNA control
When the researchers disabled the microRNA gene, they also found that cellular iron uptake was impaired due to reduced expression of the iron importer TfR1. Red blood cells and their precursors demand large amounts of iron to produce hemoglobin. Reduction of cellular iron indirectly inhibited the activity of mTORC1, thereby reducing its ability to suppress autophagy of alpha-globin, consequently alleviating beta-thalassemia symptoms.
“We unexpectedly discovered that TfR1 levels were reduced with the miR-144/451 knockout,” Weiss shared. “However, we made the connection to autophagy because we knew that other methods to deprive red cells of iron are being analyzed as a treatment for beta-thalassemia and that mTORC1 is stimulated by iron.”
Thus, the microRNA appeared to regulate two different metabolic pathways that interact with mTORC1 and thereby influence control over autophagy of alpha-globin — suggesting multiple pathways for pharmaceutical intervention in beta-thalassemia.
New possibilities to repurpose drugs for beta-thalassemia
“AMPK and mTORC1 are key metabolic sensors in a cell,” Weiss said. “Our findings solidify a connection between beta-thalassemia and its regulation by variations in metabolism that are sensed by AMPK and mTORC1, which are likely to be amenable to therapeutic intervention.”
Given the study’s results, some drugs developed for other diseases may be repurposed to treat beta-thalassemia by increasing autophagy of alpha-globin. Approved drugs for mTORC1 inhibition already exist, and drugs to activate AMPK or ULK1 are under development.
Rapamycin, a well-known mTORC1 inhibitor, improves beta-thalassemia symptoms. However, it can have severe side effects, which can be life-threatening at high doses. Bone marrow transplantation and gene therapy cure beta-thalassemia, but these treatments are risky and expensive. Weiss, Lechauve and their teams are looking for alternatives that can alleviate beta-thalassemia, particularly milder forms of the disease in which the risk of curative therapies outweighs the potential benefit, or in low- and middle-income countries, where the disease is most common and curative therapies are not yet feasible to administer.
“Any drug we find won’t be as potent as gene therapy,” Lechauve acknowledged, “But beta-thalassemia often affects individuals from low- and middle-income countries who can’t afford these treatments. If we can develop a medicine that’s both affordable and easily accessible, it may not cure the disease, but it can significantly improve the health of some patients.”
Applications for other protein aggregation diseases
The research also hints at possible therapeutic approaches for other protein aggregation diseases.
“In addition to beta-thalassemia, many diseases such as Parkinson’s, Alzheimer’s and Huntington’s have protein aggregates that cause pathology,” Weiss explained. “Stimulating autophagy had therapeutic effects in our beta-thalassemia model, which may be true for these other protein aggregation disorders as well.”
While the current study did not directly address these other diseases, it suggests a proof-of-concept for other protein aggregation disorders. Researchers have long believed many of these disorders may benefit from increasing autophagy of the built-up toxic proteins. However, many protein aggregation disorders affect neurons, a long-lived cell type that rarely renews, making it challenging to model and treat. In contrast, red blood cells turn over relatively rapidly and, therefore, have the potential to respond quickly to therapies that stimulate the elimination of toxic alpha-globin.
“Our work supports the general principle that therapeutic manipulation of protein quality control mechanisms can be effective for many protein aggregation disorders,” Weiss concluded. “In this way, studies to accelerate alpha-globin degradation in beta-thalassemia could benefit other diseases.”
The study’s co-first authors, Julia Keith and Georgios Christakopoulos, MD, both of St. Jude, alongside Lechauve, were instrumental in recognizing and following the serendipitous connections that led to making these discoveries. They helped to put the different puzzle pieces of data together, then arranged them to see the “big picture” of miR-144/451’s role in beta-thalassemia and autophagy.






