St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Gene therapy at St. Jude
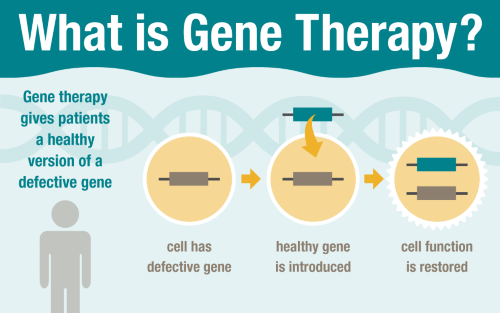
Gene therapy treats genetic diseases by giving patients a healthy version of a defective gene. St. Jude is developing innovative gene therapy approaches for patients with blood diseases, immune disorders and other conditions.
Potential for a cure
A baby is born with a defective gene, leading to a life-threatening disease. Standard therapies are limited; the child is faced with battling a chronic condition for life.
But perhaps there is an alternative — one that may lead to a permanent cure.
Gene therapy is designed to restore the function of a patient’s defective gene by introducing a healthy copy, with the potential to permanently correct a genetic disease. This compelling concept has intrigued the medical community for more than 20 years. While still primarily performed as part of research studies, gene therapy is becoming more widely adopted for clinical treatment of some conditions.
Bubble boy disease
For children born with severe combined immunodeficiency (SCID), a parent’s touch can be life-threatening. Often called “bubble boy disease,” SCID devastates the immune system and leaves patients vulnerable to lethal infections. Early death is common, even with existing therapies.
X-linked SCID, the most common form of SCID, is caused by defects in a gene encoding a critical protein called the common gamma chain. Without it, immune system cells called T cells, B cells and natural killer cells do not develop normally.
With the aim of pioneering a safe, effective gene therapy approach, St. Jude scientists have spent years developing an innovative vector for X-linked SCID. The vector is manufactured in the on-site Good Manufacturing Practice (GMP) facility using a process developed at St. Jude. The process, which uses stable cultured cell lines to produce the vector, addresses the challenge of manufacturing large quantities of clinical-grade vector in a reproducible manner.
The vector has been designed with features to reduce the risk of activating cancer-causing genes. It has also been subjected to extensive laboratory testing to ensure it does not readily insert into chromosomes near such genes.
Clinical trials led by St. Jude and the National Institute of Allergy and Infectious Diseases (NIAID) have recently confirmed that this vector can safely provide long-lasting health benefits to patients with X-linked SCID.
Read about the St. Jude trial: LVXSCID-ND: Gene Transfer for X-Linked Severe Combined Immunodeficiency in Newly Diagnosed Infants
Featured research: Gene therapy provides cure for SCID
St. Jude researchers have cured X-linked SCID in eight infants treated at St. Jude and UCSF Benioff Children’s Hospital San Francisco. As of early 2019, eight infants have received the novel combination therapy and have not shown any immediate side effects. The patients have begun developing healthy immune systems and have started responding to vaccinations. This study also demonstrated that the gene-corrected cells could be produced reliably and in significant quantities to treat a large number of patients.
Read full results published in the New England Journal of Medicine.
Learn more about the study.
Gene therapy offers hope of a cure
Scientists at St. Jude developed a novel gene therapy for children born with a rare immune disease
Gene therapy for hemophilia and other blood disorders
In 2014, St. Jude investigators and colleagues published historic results from a gene therapy trial for hemophilia B. A vector developed and manufactured at St. Jude had transformed the lives of young men with this inherited bleeding disorder.
Andrew M. Davidoff, MD, St. Jude Children's Research Hospital
Hemophilia B is caused by defects in the gene for factor IX, a blood clotting protein. Hemophilia B can be managed with lifelong regular injections of factor IX protein, which can cost $250,000 per year.
Years after receiving a single dose of gene therapy, patients on the hemophilia B trial continued to produce their own clotting factor from the normal transferred gene with minimal side effects. The treatment dramatically decreased their need for protein replacement injections, and some were able to participate in sports without worrying about bleeding.
The trial stemmed from a decade-long collaboration between St. Jude and University College London to discover effective gene therapies for this blood disorder. The St. Jude team was led by Andrew Davidoff, MD, chair of St. Jude Surgery and Arthur Nienhuis, MD, of St. Jude Hematology. Vector development was a critical aspect of the project, with years of careful improvements required to produce sufficient levels of factor IX to relieve symptoms.
Read about the hemophilia B study.
St. Jude and University College London are currently collaborating on another Phase I/II gene therapy clinical trial for adult men with hemophilia B, scheduled to launch in the U.S. in early 2019.

Andrew M. Davidoff, MD, St. Jude Children's Research Hospital
Designing better gene therapy vectors
A major step forward has been the development of safer approaches following lessons learned from early clinical trials. In gene therapy, new genetic material (DNA or RNA) is introduced into a patient’s cells, most often via a disabled virus called a vector. The new genetic material either floats free in the cells or is inserted into the patient’s chromosomes, depending on the type of vector.
St. Jude has played a key role in vector development, pioneering innovative vector designs for patients with hemophilia and the devastating immune disorder X-linked severe combined immunodeficiency (SCID). Other vectors are in development. St. Jude has also developed exclusive manufacturing processes to produce large amounts of clinical-grade genetic material for clinical trials.
Vectors designed and manufactured at St. Jude have already helped transform the lives of patients participating in gene therapy clinical trials for immune and blood disorders.
Challenges and opportunities
To date, results from clinical studies of gene therapies pioneered at St. Jude have been promising. It will be important to follow patients for many years to confirm the therapy’s long-lasting safety and health benefits.
St. Jude researchers are now focused on applying investigational gene therapy approaches to treat X-linked SCID, hemophilia B and a related disorder, hemophilia A. The long-term vision is to apply these technologies to other genetic diseases, such as sickle cell disease, and to explore applications in developing effective immune therapies for cancer.

References
Mamcarz E, Zhou S, Lockey T, Abdelsamed H, Cross S, Kang G, Ma Z, Condori J, Dowdy J, Triplett B, Li C, Maron G, Sorrentino B, et al. Lentiviral Gene Therapy Combined with Low-Dose Busulfan in Infants with SCID-X1. N Engl J Med 380:1525-1534, 2019. doi: 10.1056/NEJMoa1815408.
De Ravin SS, Wu X, Moir S, Kardava L, O’Brien SA, Ulrick J, Kwatemaa N, Little P, Theobald N, Choi U, Su L, Marquesen M, Hilligoss D, Lee J, Buckner CM, Zarember KA, O’Connor G, McVicar D, Kuhns D, Throm RE, Zhou S, Notarangelo LD, Hanson IC, Cowan MJ, Kang E, Hadigan C, Meagher M, Gray JT, Sorrentino BP, Malech HL. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med 8(335):335ra57, 2016. doi: 10.1126/scitranslmed.aad8856
Zhou S, Fatima S, Ma Z, Wang YD, Lu T, Janke LJ, Du Y, Sorrentino BP. Evaluating the Safety of Retroviral Vectors Based on Insertional Oncogene Activation and Blocked Differentiation in Cultured Thymocytes. Mol Ther March 9, 2016. Epub ahead of print. doi: 10.1038/mt.2016.55. PMID: 26957223.
Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D, Riddell A, Pie J, Rangarajan S, Bevan D, Recht M, Shen YM, Halka KG, Basner-Tschakarjan E, Mingozzi F, High KA, Allay J, Kay MA, Ng CY, Zhou J, Cancio M, Morton CL, Gray JT, Srivastava D, Nienhuis AW, Davidoff AM. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 371(21):1994-2004, 2014. doi: 10.1056/NEJMoa1407309. PMID: 25409372. PMCID: PMC4278802.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O'Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365(25):2357-65, 2011. doi: 10.1056/NEJMoa1108046. PMID: 22149959. PMCID: PMC3265081.
Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M. 20 years of gene therapy for SCID. Nat Immunol 11(6):457-60, 2010. doi: 10.1038/ni0610-457. PMID: 20485269.
Throm RE, Ouma AA, Zhou S, Chandrasekaran A, Lockey T, Greene M, De Ravin SS, Moayeri M, Malech HL, Sorrentino BP, Gray JT. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood 113(21):5104-10, 2009. doi: 10.1182/blood-2008-11-191049. PMID: 19286997. PMCID: PMC2686181.