St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
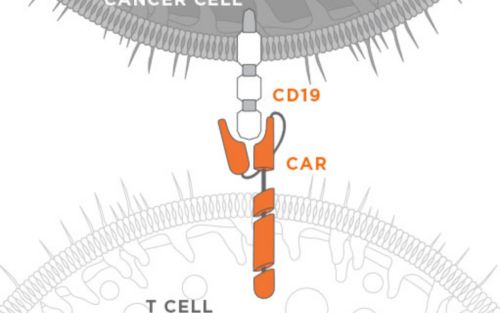
Researchers at St. Jude Children’s Research Hospital tirelessly and brilliantly work to advance cures for, and prevent, pediatric catastrophic diseases through research and treatment; and in their work they make discoveries beyond our core mission.
These discoveries require further development, so our office endeavors to license them to companies who will turn them into successful products. Here are just a few examples of discoveries licensed from St. Jude that have been incorporated into products or processes that are now deployed in the marketplace.

-
-
-
-
-
-
-
-
-
-
-