St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Understanding critical pathways drives targeted therapy efforts

Developing new therapies begins by mapping key cellular processes, pinpointing vulnerabilities and designing drugs to target them. At St. Jude, scientists drive this process from start to finish.
The path from scientific discovery to clinical application is a journey marked by winding roads, unforeseen detours and unimaginable possibilities. Along the way, each new insight is like a key, though what it can unlock often remains hidden until the right door appears. Sometimes a single discovery opens an entire landscape of therapeutic opportunity, whereas other times, progress stalls when scientific doors become jammed.
This journey is exemplified by the work of scientists who, step by step, uncover the molecular culprits behind cancer, decode their mechanisms and find ways to intervene. At St. Jude, researchers follow this path every day, from curiosity to cure, reshaping how we understand and treat cancer.
Targeting the cell cycle
Cancer is a disease of cell cycle dysregulation. The cell cycle ensures that cells grow and divide when necessary, but this control fails in most cancers, leading to unchecked cell proliferation and tumor formation. Disruptions in key regulatory proteins often drive this loss of control. Among these, CDK4 and CDK6 (CDK4/6) play a central role in governing the transition from the G1 phase, when cells prepare for DNA replication, to the S phase, where DNA synthesis occurs.
Foundational work by Charles Sherr, MD, PhD, and Martine Roussel, PhD, St. Jude Department of Tumor Cell Biology, including the discovery of mammalian D-type cyclins and CDK4, published in Cell, has been critical in uncovering how cyclin D and CDK4/6 regulate this transition. Building on these discoveries, Roussel and her team applied these insights to the study of pediatric brain cancers, particularly medulloblastoma. They developed many models of this disease, including a collection of patient-derived orthotopic xenograft (PDOX) models using primary tumor samples from St. Jude patients, published in Acta Neuropathologica. These models represent the most aggressive forms of the disease and preserve the biology of the original tumors, critical for studying pediatric brain cancers. Many of these models have proven invaluable for preclinical research and have directly guided clinical trials at St. Jude (SJMB12, SJDAWN and SJELIOT).
“Using PDOX models allows us to study ‘avatars’ when testing preclinical therapies, including CDK4/6 inhibitors in combination with other promising drugs,” said Roussel.
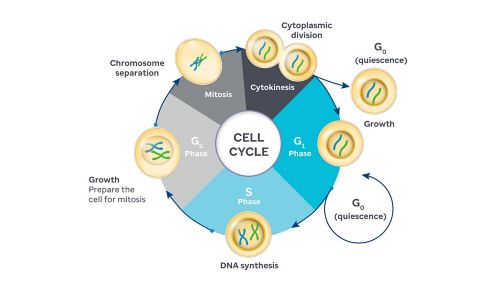
Work by Charles Sherr, MD, PhD, and Martine Roussel, PhD, St. Jude Department of Tumor Cell Biology, revealed how cyclin D and CDK4/6 control the critical transition from G1 to S phase in the cell cycle. Roussel’s team applied these insights to develop advanced models of aggressive pediatric brain cancers and test novel therapies in preclinical trials.
Decoding the complexities of p53
Once a regulatory molecule has been discovered, the next step is understanding how it functions within the broader cellular network. This task can be extremely challenging, especially in complex systems such as the cell cycle. Gerard Zambetti, PhD, St. Jude Department of Pathology, helped tease out mechanisms in the complex regulatory network surrounding p53, one of the most critical tumor suppressors. Often referred to as the “guardian of the genome,” p53 is a tumor suppressor protein that prevents cancer by repairing damaged DNA, stopping cell division or triggering cell death when genetic damage is too severe.
Zambetti’s group studies pathogenic variants in TP53, the gene encoding p53, and how they disrupt its protective role and increase cancer risk. “TP53 is the most frequently mutated gene across human cancers, altered in roughly 50% of cases. In the remaining half, other mechanisms often inactivate p53,” explained Zambetti.
Through a long-term collaboration with Raul Ribeiro, MD, St. Jude Department of Oncology, studying adrenocortical carcinoma, a rare pediatric cancer originating in the adrenal cortex. Their work, published in Proceedings of the National Academy of Sciences, led to a series of studies in Brazil, where researchers uncovered an inherited TP53 “founder” mutation in families with children affected by the disease. This novel inherited mutation is associated with an attenuated form of Li-Fraumeni Syndrome, a hereditary condition in which individuals carry germline TP53 mutations and face a high risk of developing different types of cancers and multiple tumors at a young age.
“We identified a germline p53 mutation found in roughly one in every 375 individuals from southern Brazil, a region with over 100 million people,” said Zambetti. “This unexpected discovery revealed a significant public health concern and underscored how rare cancer research can uncover broader genetic risks.”
This layered understanding of p53, its frequent mutation, inherited dysfunction and difficulty in direct therapeutic targeting, has made it one of the most challenging targets in cancer biology.
Turning cell cycle insights into therapies
Once researchers have a target and thoroughly understand the mechanism that underlies its role in cancer, it would seem the hard part is complete, and a new therapy should follow quickly. But translating those discoveries into therapies is a complex feat, with many more obstacles in researchers’ path.
CDK4/6 presented unique challenges for scientists, demanding a distinct strategy to turn mechanistic insight into clinical progress. “CDK4/6 inhibitors, they didn’t show much promise on their own, so they were set aside for a while,” explained Roussel.
CDK4/6 inhibitors could halt the cell cycle in the G1 phase and temporarily suppress tumor growth, but their effects were often reversible, and resistance quickly developed. However, advances in studies with adult breast cancer revealed that combining CDK4/6 inhibitors with other therapies could produce more promising results, paving the way for similar strategies in pediatric brain tumors.
A preclinical study published in Molecular Cancer Therapeutics showed that combining CDK4/6 inhibitors with gemcitabine, a DNA synthesis blocker, produced compounding effects in medulloblastoma models. Because the CDK4/6 inhibitor ribociclib can cross the blood-brain barrier (a physical barrier in the body that stymies many drugs from reaching the brain), it emerged as a promising option for treating brain tumors. These findings led to the St. Jude SJDAWN clinical trial, which tested ribociclib plus gemcitabine in patients with recurrent medulloblastoma.
Bridging p53 insights and clinical progress
The role of p53 in cancer is multifaceted, varying not only between tumor types but even within the same cancer. As Zambetti explains, “there are countless ways to approach targeting p53 across different cancers. Even within a single cancer type, p53 can be affected in multiple ways, and that’s just considering p53 itself.”
Because mutant forms of p53 can lose their protective function or even gain new cancer-promoting abilities, restoring or reactivating their normal activity has proven remarkably difficult. Together, these challenges highlight how even the most well-understood pathways can defy straightforward solutions on the road from discovery to treatment.
Building on decades of foundational research, Zambetti and his team are exploring ways to target specific TP53 mutations for cancer therapy. Using models of Li Fraumeni Syndrome to test small molecules that target specific TP53 variants of interest, they are looking for potential therapies that can help prevent cancer in individuals with this syndrome. The work exemplifies how decades of research can guide the search for therapies against even the most challenging molecular targets.
Discovery, treatment, prevention
From decoding fundamental mechanisms underlying key pathways, such as CDK4/6 and p53, to developing patient-derived models and testing novel therapies, this body of work illustrates the full arc of cancer research. By identifying vulnerable pathways and molecules and developing drugs to target them, researchers are turning mechanistic insights into actionable strategies that can halt tumor growth, overcome resistance and even prevent cancer in high-risk patients.






