St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
St. Jude Family of Websites
Explore our cutting edge research, world-class patient care, career opportunities and more.
St. Jude Children's Research Hospital Home

- Fundraising
Solving the mysterious case of DICER1 cancer predisposition for pediatric rhabdomyosarcoma

(L) Investigator Mark Hatley, MD, PhD, St. Jude Division of Molecular Oncology director and Department of Oncology associate member, who encountered the unsolved case of DICER1 cancer predisposition early in his career, with (R) Randy Larsen, PhD, St. Jude Graduate School of Biomedical Sciences, who solved the cold case decades later.
Research can be like a forensic investigation. When the primary culprit is cancer, it can be unclear what methods it uses to grow. Decades ago, scientists tracked children from the same families that had a significant cancer predisposition; they were much more likely than their peers to develop pediatric tumors. The why was a mystery, however. Enter investigator, Mark Hatley, MD, PhD, St. Jude Division of Molecular Oncology director and Department of Oncology associate member, who encountered the unsolved case early in his career.
“In my first project as a postdoc, I was working on understanding how these special molecules, microRNAs, contribute to cancer,” Hatley said. “Then others found there were a multitude of family members that ended up having cancer with a mutation in this microRNA processing protein, DICER1.”
MicroRNAs are molecules expressed from DNA, like a traditional gene, but they do not code for proteins. Instead, they are cut into tiny segments of RNA that bind other RNA molecules to regulate gene expression. DICER1 is the final protein that cuts immature microRNAs into their final form. People with the predisposition had lost one of their two copies of the DICER1 gene, then acquired a “second hit” mutation in the remaining gene, resulting in a DICER1 enzyme that couldn’t function correctly. Patients with the predisposition had altered gene expression, but it was unclear which genes or cells were responsible for promoting cancer.
Finding DICER1 rhabdomyosarcoma’s accomplice
While other scientists took up the case, Hatley moved to studying pediatric cancer, specifically rhabdomyosarcoma, a muscle and soft tissue malignancy. However, he continued to watch the DICER1 predisposition field develop until the evidence brought him back in.
“As we started studying these families with DICER1 cancer predisposition, we found that patients who had this germline mutation were predisposed to a multitude of different cancers, including rhabdomyosarcoma, the primary tumor that my laboratory focuses on,” Hatley explained. Hatley’s laboratory had worked out the methods for developing mouse models with their microRNA genes knocked out. This meant his team had the “cancer scene investigation” tools to find the culprit.
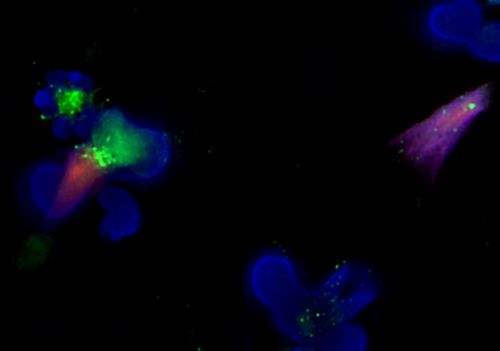
Fluorescent microscopy of neutrophils releasing Neutrophil Extracellular Traps (NETs), which appears to contribute to DICER1 cancer predisposition.
Hatley, with former St. Jude postdoctoral fellow Jason Hanna, PhD, closely examined DICER1’s impact in mouse models of rhabdomyosarcoma that mimicked the mutations found in human cases of the disease. They created a mouse that had one copy of the DICER1 gene knocked out and the other copy changed to the mutant form, both altered in its germline, so every cell contained the changes. They also created versions where an introduced tumor did or did not have these same mutations. What they found was intriguing.
“The tumor cells didn’t care,” Hatley said. When the cancer itself was mutated, there was no difference in tumor growth; however, when they only mutated the germline (inherited) cells, those mutations did promote cancer growth. “We discovered that the tumor promotion activity is not driven by the cancer, but rather by cells that were outside of the tumor, in the microenvironment,” Hatley explained.
The tumor had an accomplice.
When the scientists compared tumors that were being promoted by DICER1 mutations to those that weren’t, they found the traitorous cells: neutrophils, the most common type of white blood cells, which have many varieties. At that point, the case went cold for several years. Just like crime scene investigations before DNA evidence, the researchers lacked the tools to find the “smoking gun” that could decide the case and narrow the suspect pool.
Cracking a DICER1 rhabdomyosarcoma cold case
In the interim, single-cell RNA sequencing became more widely available, much as DNA analysis technology has become more available for forensic work. That was when Randy Larsen, PhD, St. Jude Graduate School of Biomedical Sciences, joined Hatley’s lab and dusted off the case. He went back to the scene of the crime: the tumor stroma. Stroma is the collection of cells, tissues and liquids surrounding the tumor that are not part of it. Larsen performed single-cell RNA sequencing of everything in the tumor stroma, finding, again, the enrichment of neutrophils. However, using sequencing information, he was able to go further than his predecessors to identify the cancer’s accomplice.
What he found was unexpected. Most immune cells exist on a spectrum of immature progenitor cells to mature fully differentiated cells that perform a function. Every kind of neutrophil was present, from progenitors that typically only exist in bone marrow to fully differentiated cells. Instead of a single accomplice — one specific type of neutrophil — Larsen found an entire family of neutrophils, like a criminal investigator revealing an organized crime family. He then used an antibody to remove every neutrophil in the mouse models containing the germline DICER1 mutations, showing that without any neutrophil type present, the DICER1 mutations no longer promoted cancer.
With the suspects well in hand, Larsen and Hatley needed to understand what the weapon of choice for these neutrophils was. “We went back to the sequencing data and saw an increased expression of these genes for NETosis,” Hatley said. “NETosis is a process where all of a neutrophil’s DNA decondenses and then gets shot out of the cell as a web, called a neutrophil extracellular trap, or NET, carrying proteases and other proteins out of the neutrophil to digest viruses and bacteria.”
NETosis ruptures the neutrophil, spilling these digestive enzymes into the stroma. Some of these enzymes cut through the other materials nearby, which can lead to increased blood vessel formation, a critical boon for tumor growth, providing one plausible benefit of NETs to cancer.
“Randy also showed that one of the things that gets released into the tumor microenvironment during NETosis is this peptide called CAMP,” Hately said. “It is a ligand for receptor tyrosine kinases, such as IGF1R and EGFR, which are known to have proliferative function in rhabdomyosarcoma cells.”
NET formation, releasing and creating proliferative signals near the tumor, was the smoking gun for DICER1 cancer predisposition for rhabdomyosarcoma.
Connecting DICER1 rhabdomyosarcoma evidence to humans
Armed with the knowledge of how the crime was committed, the scientist sought to prosecute their case. They used disulfiram, a drug that has already received FDA approval for other diseases. Disulfiram blocks gasdermin D from forming nuclear pores that are critical for NET formation, preventing neutrophils from rupturing and performing NETosis. When given to their mouse models, disulfiram blocked the proliferative advantage normally present in mice with DICER1 germline mutations.
While providing strong circumstantial support, the scientists still needed direct evidence that this mechanism holds true in humans. Therefore, Larsen went to the International Pleuropulmonary Blastoma/DICER1 Registry, a central pathology repository for patient tumors. He found that tumors bearing the hallmark DICER1 mutations were also enriched for neutrophil-related genes. He validated those results in the St. Jude Pediatric Cancer Genome Project and Genomes 4 Kids project RNA sequencing databases. He defended the case successfully to earn his doctorate and published the findings as a co-first author with Hanna in a recent paper in Developmental Cell.
“Nobody could have predicted this,” Hatley, co-corresponding author of the paper, said. “We were surprised by what was driving DICER1 cancer predisposition, but we’ve managed to identify a specific tumor-promoting mechanism that we can inhibit with a small molecule. In the future, if we identify the right populations of children with this predisposition, it’s possible that some sort of prevention strategy could be used to help keep tumors from forming.”
Hopefully, investigators can work together to find a way to take down this crime family at its roots — before it leads to cancer. Until then, this cold case is closed.






